Abstract
Background:
LR-MDS patients have limited treatment options and a large number are transfusion dependent (Raza A, Expert Opinion 2016, 4:9). Erythropoiesis stimulating agents (ESA) are the first line of therapy for LR-MDS patients, but the overall response rate remains low. Thus, novel strategies with the goal of red blood cell (RBC) transfusion reduction (TR) and independence (TI) are required. Rigosertib, a small molecule inhibitor of RAS effector pathways (Athuluri-Divakar SK, Cell 2016;165:643) is under investigation in MDS. The absolute bioavailability of oral rigosertib is about 35% (Komrokji, BJH 2013; 162:517). Here we expand an earlier report (Raza A, ASH 2013 #2745) on rigosertib oral in LR-MDS with updates on dose optimization on the frequency of TI or TR.
Methods:
Rigosertib oral was tested in this phase 2 study in LR (intermediate-1 or low risk per IPSS classification) transfusion-dependent MDS patients. Fifty-four (65.9%) patients had received ESA. Rigosertib was administered to RBC transfusion-dependent patients; defined as requiring a minimum of 4 packed red blood cell (PRBC) units over 8 weeks, in the cohorts shown in see Table below. Patients were eligible for efficacy analysis if they received a minimum of 8 weeks of rigosertib in their assigned dosing cohort. Erythrocyte stimulating agents (ESAs) were allowed on study. TI is defined as no PRBC transfusions over a period of 8 sequential weeks; TR a reduction in 4 or more RBC units over a period of 8 sequential weeks.
Results:
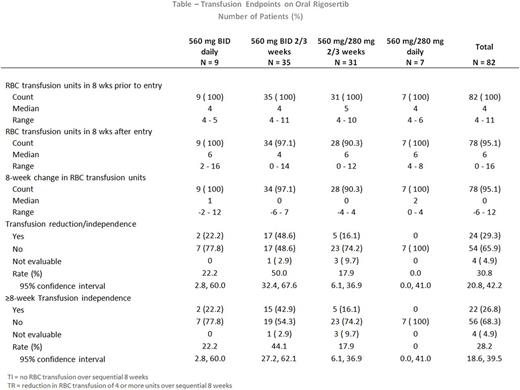
Eighty-two patients with a median age of 70 years (range 54-90) were enrolled between May 2012 and February 2015, at 5clinical sites, and received a median of 5.4 months (range 0.1-28.8) of oral rigosertib. Of the 82 enrolled patients, 9 patients were treated with 560 mg BID continuously, 7 patients treated with 560 mg in the AM and 280 mg in the PM continuously, 35 patients treated with 560mg BID intermittently, 31 patients treated with 560mg in the AM and 280mg in the PM intermittently. Sixty patients were treated with ESA and oral rigosertib while on study. An unusual adverse event (AE) (urinary events) was identified as related to dosing. Continuous rigosertib dosing cohorts were closed early due to higher urinary adverse events (AEs) or less efficacy with the lower dose of rigosertib.
Efficacy
Of the 82 patients, 66 patients received intermittent dosing for at least 8 consecutive weeks; and 20 of 62 evaluable patients (32%) achieved TI lasting 8 to 85+ weeks; with a median of 18 weeks. The highest rate of TI (44%) was observed in the 560 mg BID intermittent cohort: 15 of 34 eligible patients achieved TI lasting 8 to 85+ weeks; with a median of 18 weeks (TABLE). Ninty-three percent (93%) of these 15 patients received rigosertib with continued ESA.
Safety
Due to urinary AEs continuous 560 mg BID dosing is no longer being studied. For all intermittent patients (n=66); the most frequent treatment emergent adverse events observed were urinary with pollakiuria (42.4%), fatigue and micturition urgency (33.3%), urinary tract pain (28.8%), hematuria and dysuria (24.2%).
In the 560 mg BID intermittent group, 71.4% of all patients experienced urinary AE, the most frequent being pollakiuria (51.4%), micturition urgency (37.1%), and dysuria, hematuria, and urinary tract pain (34.3%); and fatigue (42.9%). Grade 3 or greater hematuria was observed in 11.4% of these patients and dysuria and urinary tract pain in 5.7%. Several strategies were investigated to ameliorate or manage the urinary AEs.
Notably, no significant treatment emergent myelosuppression, or other notable AEs were evident in these patients.
Conclusions:
Oral rigosertib treatment resulted in TR & TI. Patients administered rigosertib for 2 out of 3 weeks at a dose of 560 mg BID (1120 mg over 24 hrs) achieved an impressive TI rate of 44% (15/34). Based on the rate of TI, and the observed urinary AEs, the risk benefit profile of oral intermittent dosing is favorable. Oral rigosertib at a total dose of 1120 mg over 24 hrs administered intermittently in HR-MDS pts in combination with azacitidine is now being studied; with novel strategies employed to continue to minimize the unusual urinary AEs.
Raza: Kura Oncology: Research Funding; Celgene Inc.: Research Funding; Syros Pharmaceuticals: Research Funding; Janssen R&D: Research Funding; Novartis: Speakers Bureau; Genoptix: Speakers Bureau; Onconova Therapeutics: Research Funding, Speakers Bureau. Lee: Alexion Pharmaceuticals: Consultancy; Amgen: Consultancy; Clinipace: Consultancy; Baxalta: Consultancy. Ali: Kura Oncology: Consultancy; Onconova Therapeutics: Consultancy. Fruchtman: Onconova Therapeutics, Inc.: Employment. Petrone: Onconova Therapeutics, Inc.: Employment. Zbyszewski: Onconova Therapeutics, Inc.: Employment. Heaney: Novartis: Consultancy; Onconova Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal